Membrane transport is a vital physiological process that maintains cellular homeostasis, converts different energy forms, and generates and transduces signals. Based on the studies on representative channels, uniporters, and secondary active transporters, our group seeks to unveil the governing principles of membrane transport. The resolution revolution in cryo-electron microscopy (cryo-EM) has propelled structural biology into a new era. We combine cryo-EM, biochemical, and biophysical approaches to investigate the physiological and cellular processes involving membrane transport, including generation and propagation of action potentials, and cellular homeostasis of sterols. The aim to decipher the atomic choreography of these essential physiological processes and facilitate structure-based drug discovery.
Electromechanical coupling and modulation of Nav/Cav channels.
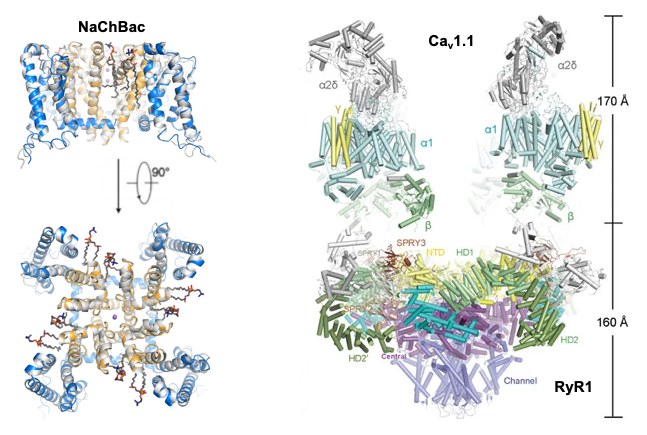
A long-term major focus in our lab is the structural elucidation of voltage-gated sodium/calcium channels (Nav/Cav) and calcium-release channels (RyR) that are essential for the physiological processes involving electrical signaling, such as the excitation-contraction coupling (ECC) of muscles. Nav and Cav channels are responsible for the initiation and propagation of electrical signaling in excitable cells such as neurons and muscle cells, while RyRs play key roles in translating electrical signals into secondary signaling responsible for myocyte contraction. Aberrant function of these channels is associated with a broad spectrum of disorders, such as pain syndrome, autism, and cardiovascular disease. These channels are targeted by many FDA-approved drugs and more are being developed. It is, therefore, essential to understand the function and disease mechanism of these channels at an atomic level, which will significantly aid future drug discovery. Our previous work has achieved the structural elucidation of eukaryotic Nav, Cav, and RyR channels, including those from the American cockroach, electric eel, rabbit muscle, and even human. To date, we have published structures of Nav1.2, Nav1.4, Nav1.7, Cav1.1, Cav1.3, rRyR1, and pRyR2 in detergent micelles, in the presence of various modulators. Our future work will address the unique changes to the channel structure in near-to-native lipid environments and address their functions in the presence and absence of FDA-approved drugs. The ultimate goal is to elucidate the structure-function relationship of the ion channels in situ using cryo-FIB and cryo-ET, which may uncover new layers in the molecular dynamics and provide new insights into novel drug design targeting specific channels.
Structural and mechanistic investigation of sterol homeostasis.
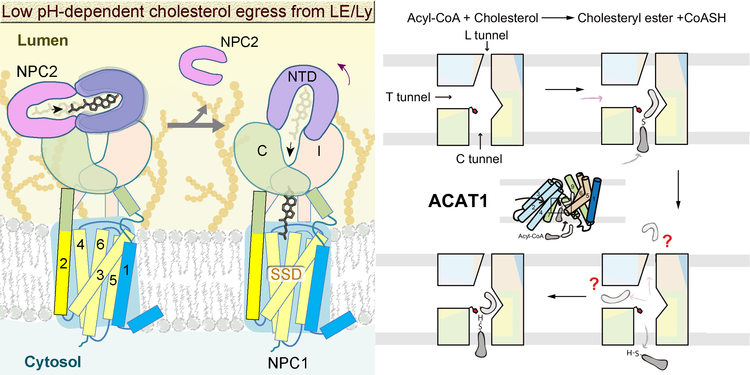
Cholesterol is an important component of mammalian cell membrane, and also serves as the precursor for steroid hormones, bile acids, and vitamin D, thereby playing important roles in hormonal regulation, lipid absorption, calcium homeostasis, and so on. However, the high level of cholesterol in the body will lead to high risk of cardiovascular disease. Therefore, the cholesterol should be kept at a reasonable level in the body. The cholesterol in the cell can come from two sources, the de novo biosynthesis through the mevalonate pathway and the LDLR mediated endocytosis of LDL. The releasing of cholesterol in the endocytic LDL requires a well-known lysosomal membrane protein NPC1, whose dysfunction will lead to Niemann Pick disease. The import and synthesis of cholesterol are tightly controlled through a classic SREBP pathway to keep the cholesterol homeostasis. Excess cholesterol is also transferred to ester form to store in lipid droplets catalyzed by ACAT membrane enzymes. Multiple membrane proteins are involved in the cholesterol homeostasis, while their molecular mechanisms are still unknown. We have solved the structures of NPC1 and ACAT1 after solubilization in detergent micelles, providing a framework to further investigate the molecular basis of their functions. We are combining multiple approaches to investigate the dynamics of these physiologically and pathophysiologoically crucial proteins

